Practicalities
We are a diverse community of students, researchers, educators, and knowledge keepers who come together to collaborate on research, share cultural and disciplinary knowledge, and exchange ideas for mutual enrichment and collective growth.
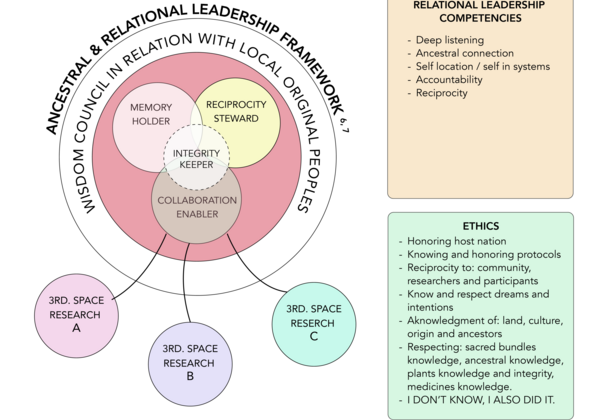
Approach
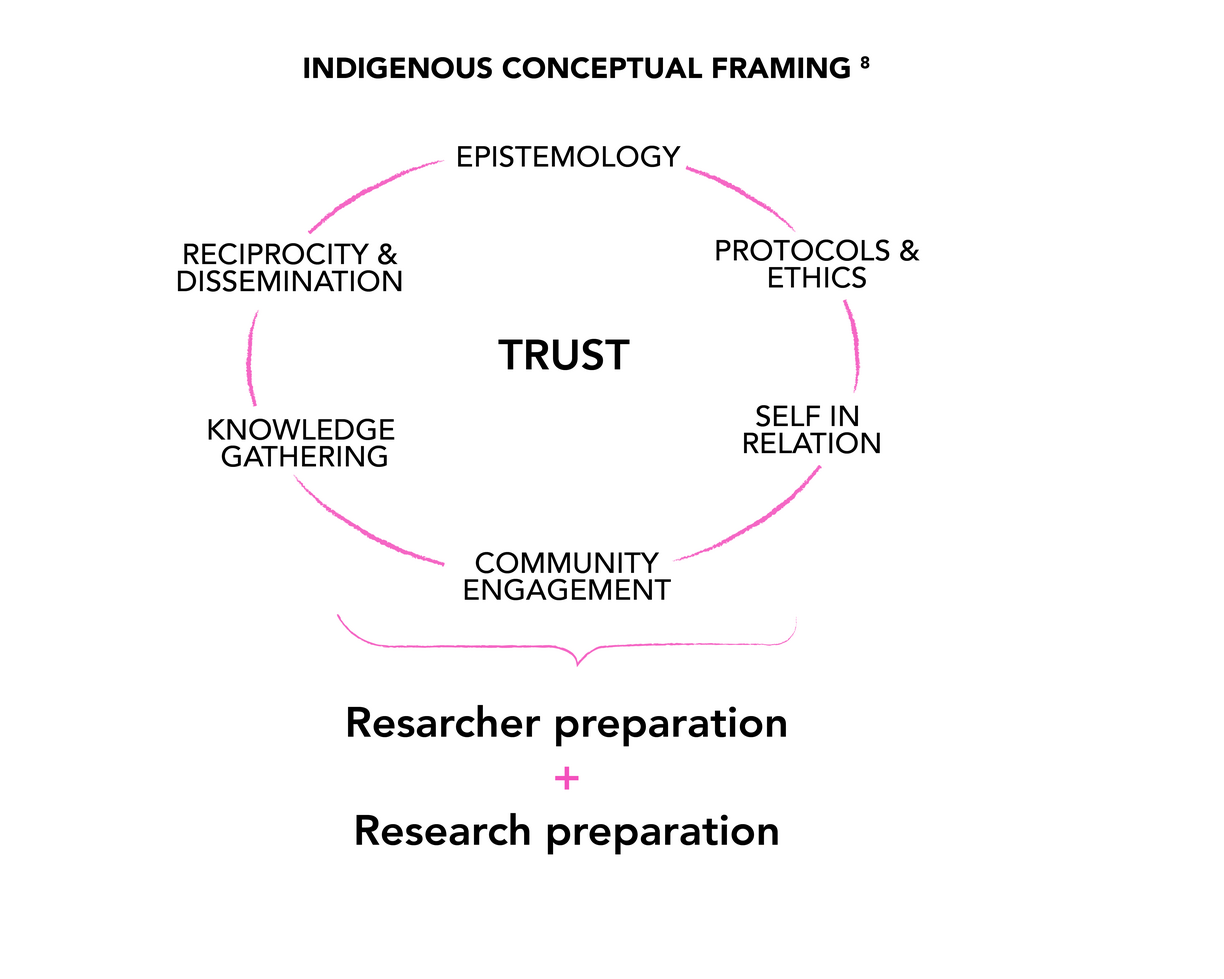
We recognize that Indigenous cultures have engaged with psychoactive medicines since time immemorial, making Indigenous ancestral knowledge an essential foundation of our work.
Despite the impact of colonization, cultural traditions have been carefully safeguarded by knowledge keepers. We strive to create an inclusive and protective space where these traditions can be honored, revitalized, and expanded.
By integrating diverse approaches, we seek to bridge the mind-body and heart-spirit spaces while upholding Elder Geraldine’s call to 'Naut sa Mawt'—to walk as one in mind and spirit. This fosters the awareness and compassion necessary for reconciliation—within ourselves, with others, and across cultural paradigms.
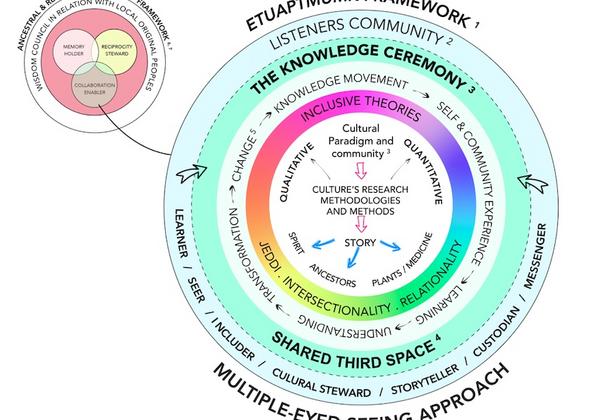
Guided by participatory action, patient-oriented research, and an Indigenous cultural safety framework, we cultivate an intercultural ‘third space’ (Bhabha, 1994; Soja, 1998)—a collaborative environment where diverse knowledge systems can interact, evolve, and benefit all.
“To see from one eye with the strength of Indigenous ways of knowing, and to see from the other eye with the strengths of Western ways of knowing, and to use both eyes together”
Albert Marshall, Mi'kmaq Elder.